Quantification of PD-L1 expression in tumor tissue using immunohistochemistry (IHC) assays is currently the most widely validated, used, and accepted biomarker to guide the selection of patients to receive immune checkpoint inhibitor (i.e., anti-PD-1 or anti-PD-L1) therapies.
Strata Oncology offers PD-L1 IHC testing alongside Strata Select testing. A single test requisition form and sample submission will provide you with comprehensive genomic profiling results and PD-L1 expression to help inform targeted therapy and immunotherapy decisions.
Benefits of Ordering PD-L1 IHC Testing with StrataNGS
-
Save time: no need to wait for block return from PD-L1 testing to submit for Strata Select testing. PD-L1 IHC results available in less than 7 days from receipt of sample at Strata Oncology
-
Conserve tissue: tissue is wasted during block preparation each time new slides are cut. A single block preparation for slides to support molecular analysis and IHC spares tissue
-
Better treatment: have a full picture of your patient’s tumor characteristics before deciding on therapy
Ordering
PD-L1 IHC testing cannot be ordered as a stand alone test. It may be ordered alongside Strata Select testing, but it is not required.
PD-L1 IHC testing, which will be billed separately, can be added to a Strata Select order by choosing the appropriate clone in either the portal or on the paper requisition form.
There are no special or additional specimen requirements for adding PD-L1 IHC testing to a Strata Select order if a block is being sent. If slides are being sent, then three (3) additional slides must be sent.
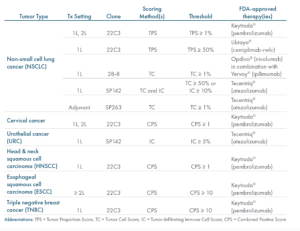
When ordering, the desired clone should be specified (see table).
If there is not enough tissue to perform both Strata Select and PD-L1 IHC testing, Strata will reach out to you and request more tissue. If this isn’t possible only Strata Select will be performed.
The Strata Oncology Financial Assistance Program is available to help commercial insurance patients, Medicaid patients and patients with no insurance with out of pocket costs.